Covid: First confirmed case of virus reinfection
- Posted By: Studio
- Coronavirus
- Updated: 25 April, 2024 08:07
- 857
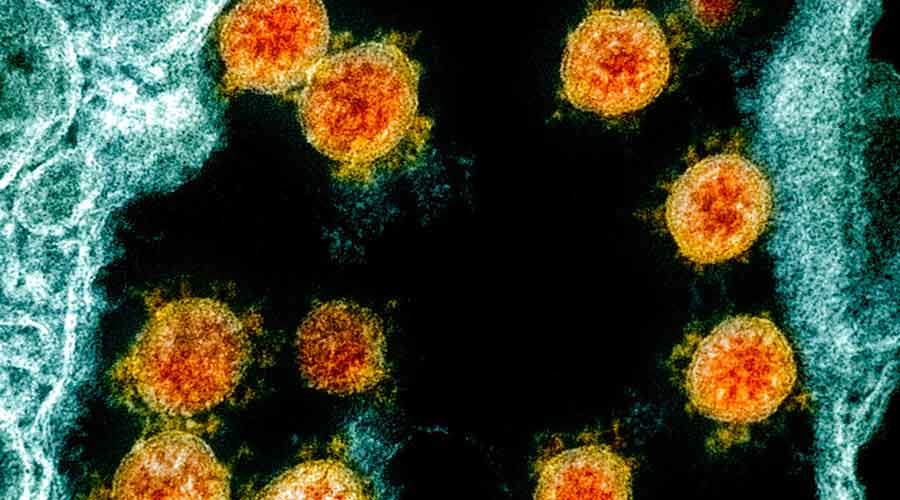
Researchers in Hong Kong are reporting the first confirmed case of reinfection with the coronavirus.
“An apparently young and healthy patient had a second case of Covid-19 infection which was diagnosed 4.5 months after the first episode,” University of Hong Kong researchers said on Monday in a statement.
The report is of concern because it suggests that immunity to the coronavirus may last only a few months in some people. And it has implications for vaccines being developed for the virus.
The 33-year-old man had only mild symptoms the first time, and no symptoms this time around. The reinfection was discovered when he returned from a trip to Spain, the researchers said, and the virus they sequenced closely matched the strain circulating in Europe in July and August.
“Our results prove that his second infection is caused by a new virus that he acquired recently rather than prolonged viral shedding,” said Dr Kelvin Kai-Wang To, a clinical microbiologist at the University of Hong Kong.
Given that there are millions of cases worldwide, it is not unexpected that a few, or even a few dozen, people might be reinfected with the virus after only a few months, experts have said.
Doctors have reported several cases of presumed reinfection in the US and elsewhere, but none of those cases have been confirmed with rigorous testing. Recovered people are known to shed viral fragments for weeks, which can cause tests to show a positive result in the absence of live virus.
But the Hong Kong researchers sequenced the virus from both rounds of infection and found significant differences in the two sets of virus, suggesting that the patient was infected a second time.
Common cold coronaviruses are known to cause reinfections in less than a year, but experts had hoped that the new coronavirus might behave more like its cousins Sars and Mers, which seemed to produce longer-lasting immunity of a few years.
The Food and Drug Administration on Sunday gave emergency approval for expanded use of antibody-rich blood plasma to help hospitalised coronavirus patients, allowing Trump, who has been pressuring the agency to move faster to address the pandemic.
Trump cited the approval, which had been held up by concerns among top government scientists about the data behind it, as welcome news in fighting a disease that has led to 176,000 deaths in the US and left the nation lagging far behind most others in the effectiveness of its response.
At a news briefing, he described the treatment as “a powerful therapy” made possible “by marshalling the full power of the federal government”.
The decision will broaden use of a treatment that has already been administered to more than 70,000 patients. But the FDA cited benefits for only some patients. And, unlike a new drug, plasma cannot be manufactured in millions of doses; its availability is limited by blood donations.
Trump urged everyone who has recovered from the virus to donate plasma, saying there is a nationwide campaign to collect it. Trump has portrayed his demands to cut red tape and speed approval of treatments and vaccines as a necessary response to a public health emergency.
But Sunday’s announcement came a day after he repeated his unfounded claim that the FDA was deliberately holding up decision-making until after the election, this time citing a “deep state”.
That accusation exacerbated concerns among some government scientists, outside experts and Democrats that the President’s political needs could undermine the integrity of the regulatory process.








Comments